SO2 Lewis Structure: Simplified Diagram & Explanation

Understanding the SO2 Lewis Structure is essential for anyone studying chemistry, particularly in the context of molecular geometry and chemical bonding. Sulfur dioxide (SO2) is a significant compound with applications in various industries, including air pollution control and food preservation. This blog will guide you through the SO2 Lewis Structure, providing a simplified diagram and a detailed explanation to help you grasp its fundamentals.
What is the SO2 Lewis Structure?

The SO2 Lewis Structure is a diagram that represents the arrangement of atoms and electrons in a sulfur dioxide molecule. It helps visualize how sulfur (S) and oxygen (O) atoms bond and share electrons. Lewis structures are crucial for predicting molecular shape, polarity, and reactivity.
Step-by-Step Guide to Drawing the SO2 Lewis Structure
Count the Total Valence Electrons
Sulfur (S) has 6 valence electrons, and each oxygen (O) atom has 6 valence electrons. Since SO2 has one sulfur and two oxygen atoms, the total is:
(6 (S) + 2 \times 6 (O) = 18) valence electrons.Determine the Central Atom
Sulfur (S) is the central atom because it is less electronegative than oxygen.Connect the Atoms with Single Bonds
Draw a single bond between sulfur and each oxygen atom. This uses up 4 electrons (2 bonds).Complete the Octets
Place the remaining 14 electrons around the atoms to complete their octets. Each oxygen atom will have 6 electrons (3 lone pairs), and sulfur will have 2 lone pairs.Check for Formal Charges
Calculate formal charges to ensure the structure is stable. The most stable structure has a double bond between sulfur and one oxygen atom, with the other oxygen having a single bond and 3 lone pairs.
📌 Note: The SO2 Lewis Structure has a bent molecular geometry due to the lone pairs on sulfur, which cause electron repulsion.
Simplified Diagram of SO2 Lewis Structure

Below is a simplified representation of the SO2 Lewis Structure:
| Atom | Bonding | Lone Pairs |
|---|---|---|
| Sulfur (S) | 1 double bond, 1 single bond | 2 lone pairs |
| Oxygen (O)1 | 1 double bond | 2 lone pairs |
| Oxygen (O)2 | 1 single bond | 3 lone pairs |

Key Takeaways
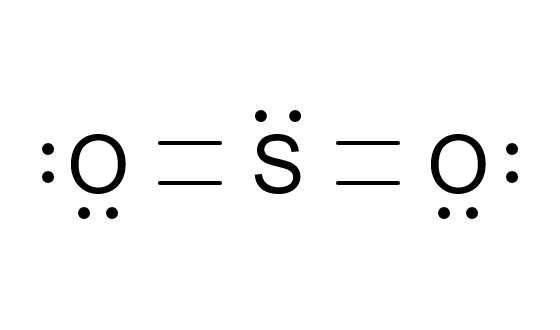
- The SO2 Lewis Structure consists of one sulfur atom bonded to two oxygen atoms.
- One oxygen atom forms a double bond with sulfur, while the other forms a single bond.
- Sulfur has two lone pairs, contributing to the molecule’s bent shape.
- Understanding this structure is vital for predicting SO2’s chemical properties and reactions.
For those looking to deepen their knowledge, exploring related topics like molecular geometry, VSEPR theory, or chemical bonding can provide additional insights.
What is the molecular geometry of SO2?
+The molecular geometry of SO2 is bent due to the lone pairs on the sulfur atom causing electron repulsion.
Why does SO2 have a double bond?
+SO2 has a double bond to minimize formal charges and achieve a more stable electron configuration.
How does the SO2 Lewis Structure relate to its polarity?
+SO2 is polar due to its bent shape and the difference in electronegativity between sulfur and oxygen atoms.
By mastering the SO2 Lewis Structure, you’ll gain a foundational understanding of molecular structures and their implications in chemistry. Whether for academic purposes or practical applications, this knowledge is invaluable.
Related Keywords: SO2 molecular geometry, Lewis dot structure, chemical bonding, VSEPR theory, molecular polarity.